Polymer & Co.
DSC
Limosolve
What is Limosolve (HIPS) ?
High Impact Polystyrene (HIPS) consists of polystyrene reinforced with polybutadiene or another unsaturated rubber. It was chosen because of its solubility in limonene.
Our objective is to use this polymer as a sacrificial base for the construction of our hand.
Physico-chemical properties



HIPS is brittle; has a high impact strength; suffers from photooxidation; and has a white color.
The Polystyrene (PS) or poly(1-phenylethylene) is a thermoplastic mostly used for the production of drinking cups and its structure is represented in figure 1. It has a glass transition (Tg) at about 100°C and it is ussualy atatic and does not crystalize .

Polybutadiene or poly(1-butenylene) is used mostly for tires and it has a glass transition of approximately -102°C. It can be cis or trans. This last one is the configuration of the commercial rubber and it is shown in figure 2.

The HIPS consists of polybutadiene (or other unsaturated rubber) dispersed in polystyrene. The morphology consists of spherical particles of polybutadiene in a polystyrene matrix as shown in the figure 1. Its synthesis consists of free radical polymerization by the bulk-bulk method.

Application field
HIPS is used for low temperature applications and offers an easy and low cost print. It is used mainly for packing and housing.



The printing material
According to the manufacturer, the maximum operating temperature of the HIPS is 50°C.
Its softening temperature is close to 90°C and the printing temperature is between 220 and 260 °C (235°C is the recommended one).
Thermal decomposition occurs only for temperatures higher than 300°C
Experimental analysis
3D-Printing test
The first object printed was a standardized test object that can be found in the NIST website [8]. The printing was performed at 235°C for the extruder; 100°C for the platform that was covered with blue tape. The material was previously put in a dryer at 120°C during 8h to get rid of all the water. One sees that the printing has a high quality, however one feels that the material is fragile. The object is shown in figure 4.


The second printing test was done in order to build a dog bone sample and a rectangle. The printing was done without problems. The setup used was the same as the first impression. The results are shown in figure 5.
A Differential scanning calorimetry was performed as a thermal analysis of our sample.
First the sample was heated until 275ºC at a rate of 10ºC/min and then cooled until 35ºC and heated again. The results are shown in figure 6.

The green line represents the first heating. This first heating is done in order to delete all the thermal background of the material. The blue line in the figure 3 represents the cooling; and the black, the second heating. One can observe, in the cooling line, a glass transition at approximately 96℃, which is compatible with the literature. This corresponds to the Tg of the polystyrene. The Tg of polybutadiene is not observed as it occurs at temperatures lower than 35℃. There are also two peaks in the heating line at almost the same temperature, due to the relaxation that occurs in the Tg.
Rheometry
The rheometry test was performed on cylindrical samples of 8 mm of diameter and 1 mm of thickness that were printed at a temperature of 235ºC. First, to calculates the linear regime and set the deformation, a strength sweep test was perfomed with constant angular frequency equals to 10 s-1. Then, a frequency sweep test was done for five different temperatures: 210ºC; 220ºC; 230ºC; 240ºC; 250ºC. The main results are shown in the figure 2.
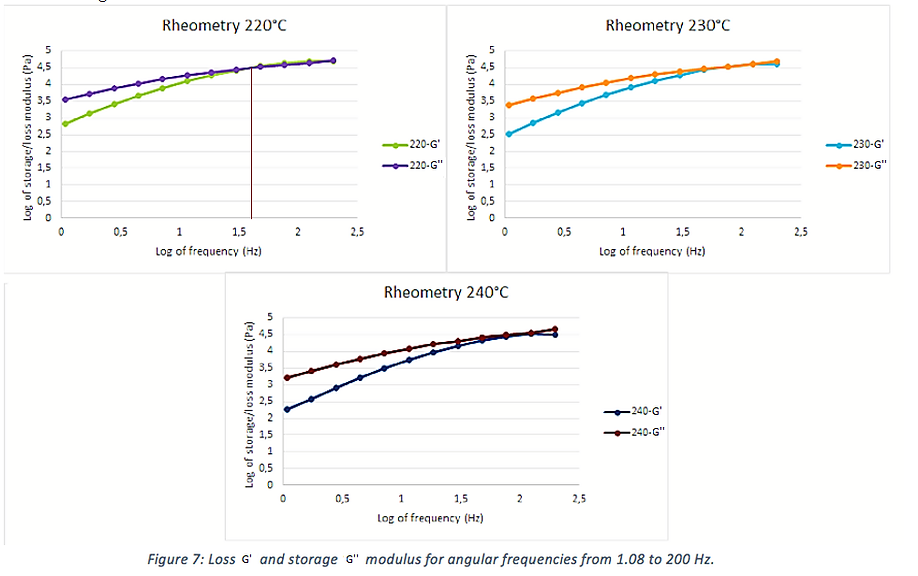
One sees that at 220°C, the polymer has a solid behavior for frequencies higher than 48 Hz, meaning a relaxation time of 0.02 seconds, approximately. This is due to the fact that at low frequencies the molecules have enough time to flow and to deform plastically. This can be correlated to the Deborah number. This number consists of the ratio between the relaxation time and the observation time. However, at temperatures higher than 230°C, limosolve has a fluid behavior for all the frequencies analyzed. With this analysis one can conclude that at temperatures higher than 220°C, the polymer has a fluid-like behavior, which is consistent with the literature data; and that for temperatures below 220°C, it has a viscous-elastic behaviour. One can also see that both the loss modulus and the storage modulus are increased when the temperature decreases.
Assuming that the material was thermorheologically simple, the graphics of different temperatures were translated by a factor at so a master curve was produced for a reference temperature of 210ºC. The translation factor was determined empirically, and the results are shown in the figure 8.
The master curve is represented in figure 9 and it shows that the material has a relaxation time of about 0,05 seconds (20 Hz), represented by the crossover of G’ and G’’. That is the necessary time for the chains to relax and disentangle, in other words, is the reputation time.



Figure 10 shows a plot of log(aT) by the reciprocal temperature. It can be seen that the for all the analyzed temperatures, the sample is explained by Arrhenius equation:
And so, the activation energy was calculate and the result is Ea=80KJ/mol.

Dissolution in limonene
A dissolution test was performed adding a bar of 2.5g of limosolve in a solution of 10 mililiters of limonene. After three hours; all the limosolve was dissolved in the limonene solution, as one sees in the figure 8 and 9.

Figure 8: limosolve is added in a limonene solution

Figure 9: all the limosolve is dissolved in the solution
Conclusion
This polymer is not good for temperatures higher than 50°C. For the printing it is interesting to print at temperatures higher than 230°C as for this case the polymer will present a fluid-like behavior. The HIPS is a good option to build supports for the other structures, since it can be easily removed, but it cannot be used for our hand, since it does not support high temperatures.
By Fernando
[1] https://www.azom.com/article.aspx?ArticleID=424
[2] https://www.formfutura.com/shop/product/limosolve-natural-250
[3] Yoo, Pyeong Jun, and Taeyoung Yun. "Micro-heterogeneous modification of an asphalt binder using a dimethylphenol and high-impact polystyrene solution." Construction and Building Materials 49 (2013): 77-83.
[4] Rieger, J. Journal of Thermal Analysis (1996) 46: 965
[5] https://en.wikipedia.org/wiki/Polystyrene
[6] Kaufman, H. S., & Falcetta, J. J. (1977). Introduction to polymer science and technology: an SPE textbook. Wiley.
[7] https://www.polymershapes.com/product/high-impact-polystyrene-hips/
[9] http://www.atomer.fr/1/1a-Tg-temperature-transition-vitreuse.html
[10] Soriano‐Corral, Florentino, et al. "Synthesis and Characterization of High Impact Polystyrene from a Heterogeneous Styrene‐Rubber‐Polystyrene Solution: Influence of PS Concentration on the Phase Inversion, Morphology and Impact Strength." Macromolecular Symposia. Vol. 325. No. 1. WILEY‐VCH Verlag, 2013.